ickplant@lemmy.world to Lemmy Shitpost@lemmy.world · 2 years agoSweet tealemmy.worldimagemessage-square165fedilinkarrow-up11.68Karrow-down175
arrow-up11.6Karrow-down1imageSweet tealemmy.worldickplant@lemmy.world to Lemmy Shitpost@lemmy.world · 2 years agomessage-square165fedilink
minus-squareTangent5280@lemmy.worldlinkfedilinkarrow-up2arrow-down1·2 years agoHow? Wouldn’t the excess sugar just come out of solution when the tea cools down again?
minus-squareraptir@lemm.eelinkfedilinkEnglisharrow-up5arrow-down1·2 years agoThey’re not super saturating it. They’re putting an amount of sugar in the tea that can dissolve at room temperature, it just takes a long time to do so.
minus-squareTangent5280@lemmy.worldlinkfedilinkarrow-up1·2 years agoOk, got it. Someone in this thread mentioned ice cold water can still hold 1.7x its weight in sugar.
minus-squarejoel_feila@lemmy.worldlinkfedilinkarrow-up1·2 years agoYeah basically, leave the pitcher to evaporate and you get your sugar back as a coating on thr glass
minus-squareTonyTonyChopper@mander.xyzlinkfedilinkarrow-up1·2 years agoIt dissolves quickly when the solution is warm. You would need to add a ridiculous amount for it to be saturated at room temp or slightly below. “ice cold” water can hold about 170 grams of sugar in 100 grams of water
How? Wouldn’t the excess sugar just come out of solution when the tea cools down again?
They’re not super saturating it. They’re putting an amount of sugar in the tea that can dissolve at room temperature, it just takes a long time to do so.
Ok, got it. Someone in this thread mentioned ice cold water can still hold 1.7x its weight in sugar.
Yeah basically, leave the pitcher to evaporate and you get your sugar back as a coating on thr glass
It dissolves quickly when the solution is warm. You would need to add a ridiculous amount for it to be saturated at room temp or slightly below.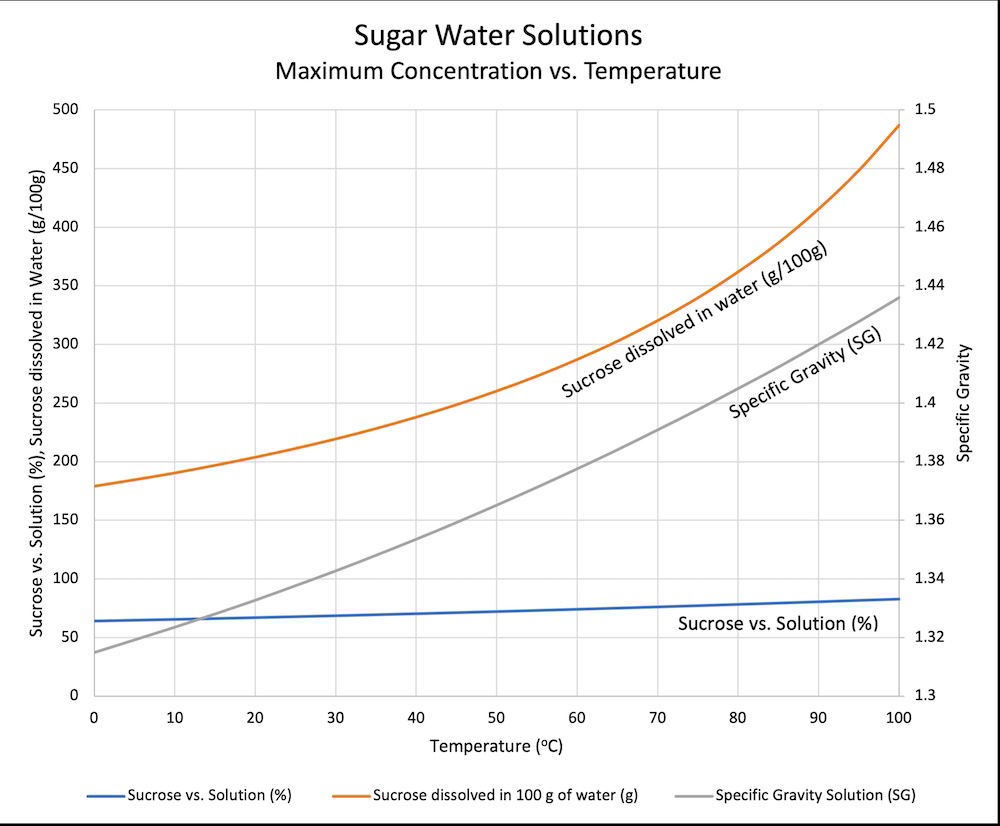
“ice cold” water can hold about 170 grams of sugar in 100 grams of water